Regulatory Consultation
Complying with regulatory requirements is the gateway to local markets everywhere in the world.
Japan is no exception. We enable our clients to take a comprehensive view of the local regulatory environment and help them put together the most efficient approach to approval.
Gap analysis
Laying out paths and timelines
Preliminary dialogues with regulatory officials
Applicability assessment of timesaving development programs
Business impact analysis of regulatory alternatives
Case
Validation of Clinical Development and Pricing Strategy
A German-based biotech in discussion with a Japanese company about the potential licensing of its product. The product targets the treatment of a disease with large unmet medical need currently receiving little treatment.
The product was finishing its Phase III program in Europe with plans to submit the application for approval to the authorities.
Evaluation of Clinical Value Over Competitors
Swiss-based pharmaceutical company seeking business opportunity to develop their product in Japan. Competitive products for the same disease already existed in the Japanese market. Client needed to assess the clinical value of their product and its potential to succeed in Japan, including any advantage over competitors.
Gap analysis
Utilizing existing data in the NDA is an effective way to meet local standards.
We carry out gap analyses to identify requirements that can be filled with documents already prepared for other applications vs. those documents that need to be created.
This strategy is particularly important when clients employ bypass methods in their own markets, such as the 505 (b)(2) pathway for FDA approval.
Laying out paths and timelines
Designing a local clinical development process that is aligned with global plans is a complicated task.
Effective planning will identify the timing of global information availability that local tasks may depend on.
In our planning process, we make sure that all tasks and processes are streamlined. We map out the entire project flow to visualize where key process bottlenecks might exist.
Preliminary dialogues with regulatory officials
In the complex world of local clinical development, it is always helpful to get both official and unofficial advice from the field in order to navigate your product through the process. We help our clients get a sense of what is acceptable in their development plans and what is not.
This feedback can save valuable time when making strategic decisions
Applicability assessment of timesaving development programs
Various regulatory paths are prepared to expedite the development of drugs with cutting edge innovation expected to solve diseases with high unmet medical need. Those include
- Priority review system: applies to drugs recognised as clinically important
- Restrictive approval system: in case of emergencies (e.g., COVID-19)
- Orphan drugs: drugs treating diseases with number of patients fewer than 50,000 and with expected clinical usefulness
- Drugs of pediatric use
- Unapproved drugs and drugs of off-label use: drugs not yet approved but with high unmet medical need
- SAKIGAKE: innovative products expected to launch first in Japan
e-Projection advises clients seeking further information on the applicability of these programs to their own drug development plans.
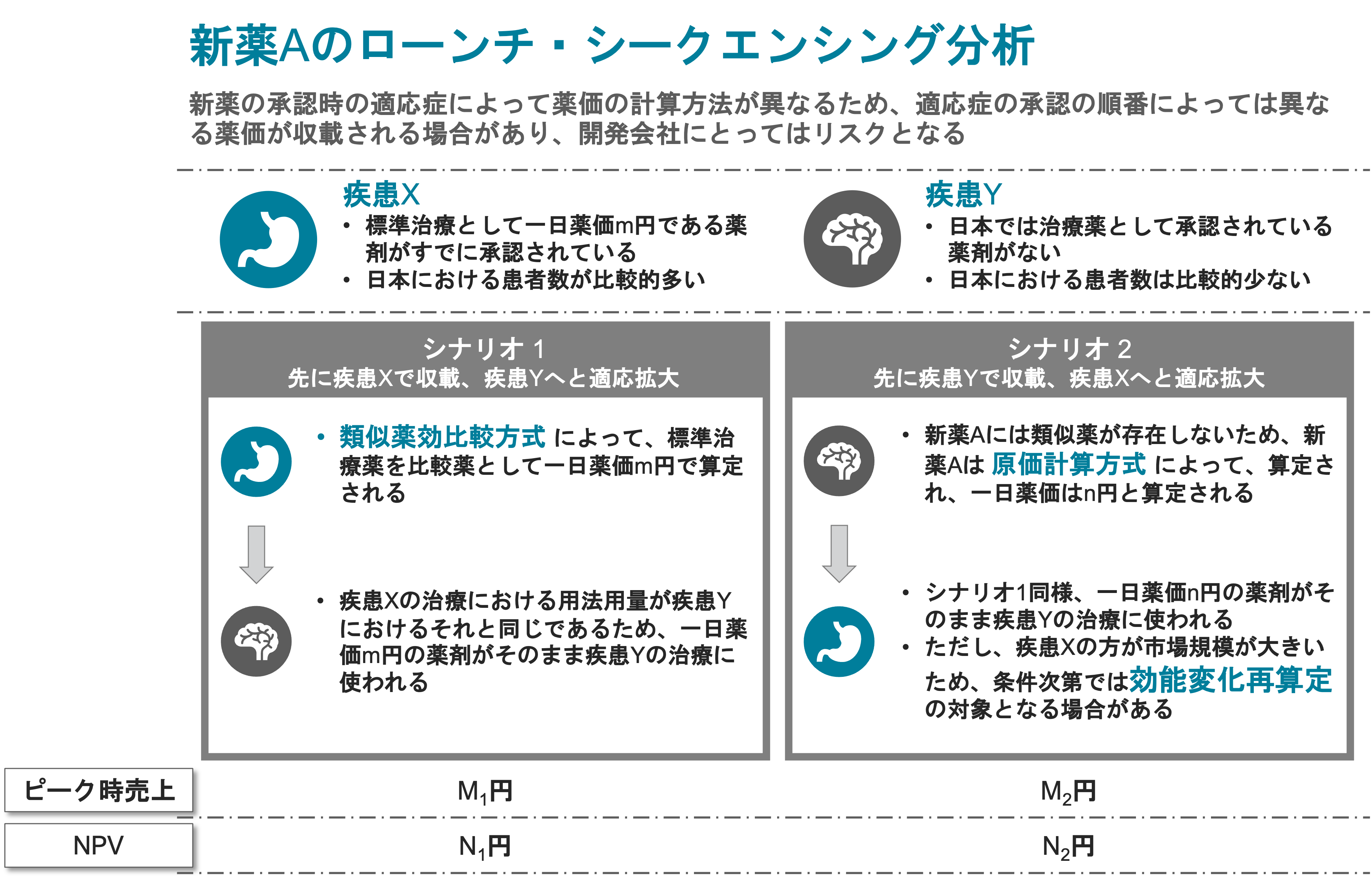
Business impact analysis of regulatory alternatives
We combine regulatory assessments with valuation capabilities to offer our clients a business planning model based on multiple regulatory scenarios.
As a result, our clients are able to understand the difference between product valuation and risk and the probability of success across the scenarios.