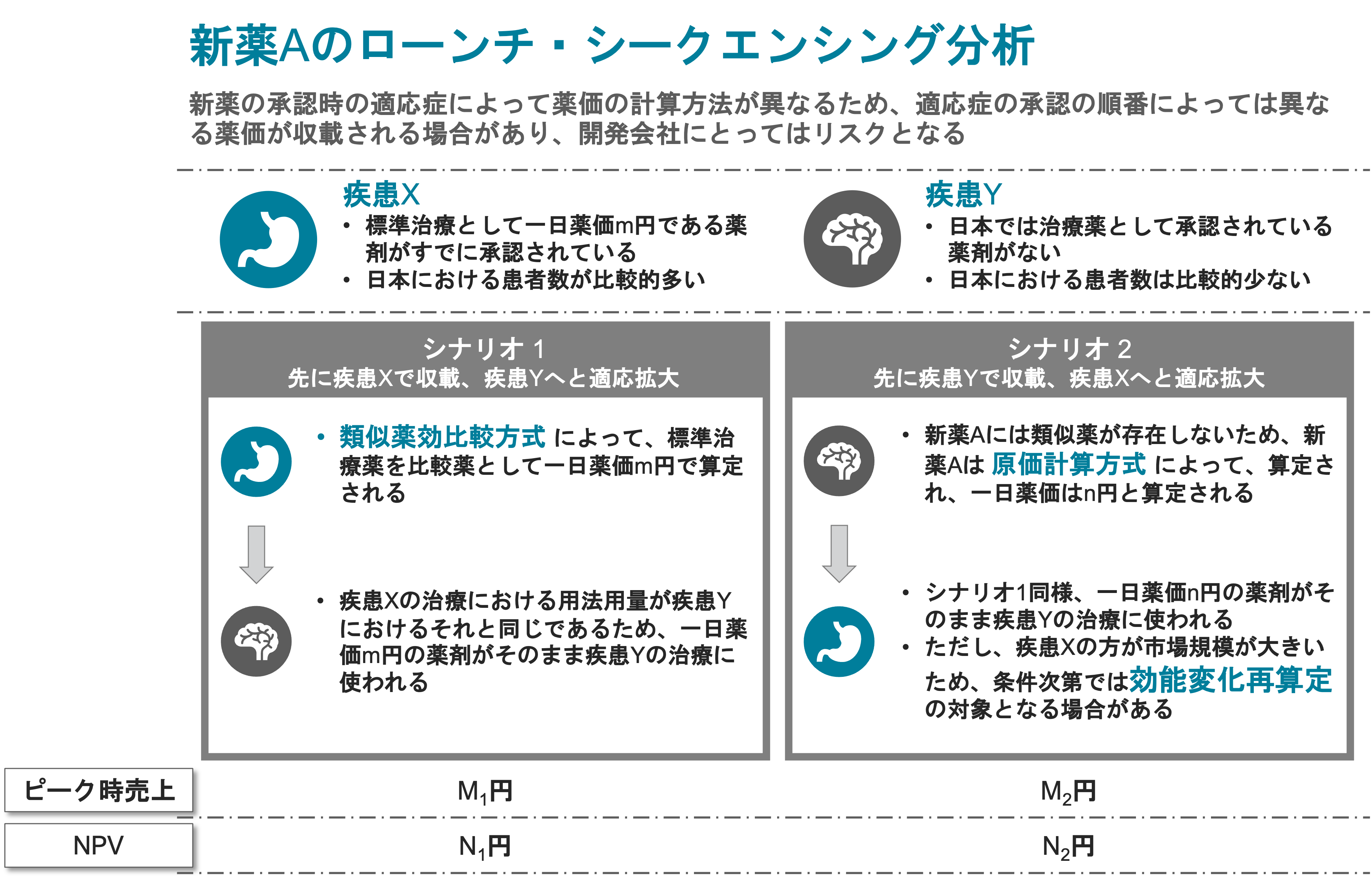
Pricing Strategy for Device and Drug Combination
Background
US-based biotech exploring how to obtain premium pricing for their medical device and drug, individually as well as their combination, for a treatment of eye diseases.
Client also sought to gain an understanding of the various ways for reimbursement of this combination procedure.
Objective
Understand means, conditions and feasibility for achieving premium pricing of the drug component.
Project Summary
-
Secondary Search
Study published documents from public and private sectors to understand precedent/similar cases, rules/regulations and tendencies of the authorities. Includes stakeholder mapping. -
Payer Interview
Review pricing strategies from viewpoint of authorities to understand challenges in the absence of a formal process specific to a combinatorial product. -
Scenario Building
Provide multiple scenarios each with timelines and consequences -
Recommendations
How best to approach the Japanese market.
Client Feedback
The solutions e-Projection provided were based on evidence and exhaustive analysis of potential commercialization.
Based on strategic scenarios for reimbursement of this combination procedure, the client now understood the key to developing the technology in the Japanese market.
Timeline
8 weeks
Key Project Capabilities
- Pricing and Market Access Consultation
- Regulatory Consultation
- Clinical Consultation
- Demand Analysis
- Market Research
Deliverables
- PowerPoint presentation
Informal interviews with government officials
Creative approaches to higher levels of value
Timelines and process maps
We help non-Japanese pharma/biotech companies
understand and expand into the Japanese market.