Japan Opportunity Assessment
Background
US-based biotech company developing phase II product in US/EU, trying to assess whether to expand development activities into Japan now or later.
In planning for an IPO in a few months time, the company needed to ensure that investors understood the true global value of their product.
Objective
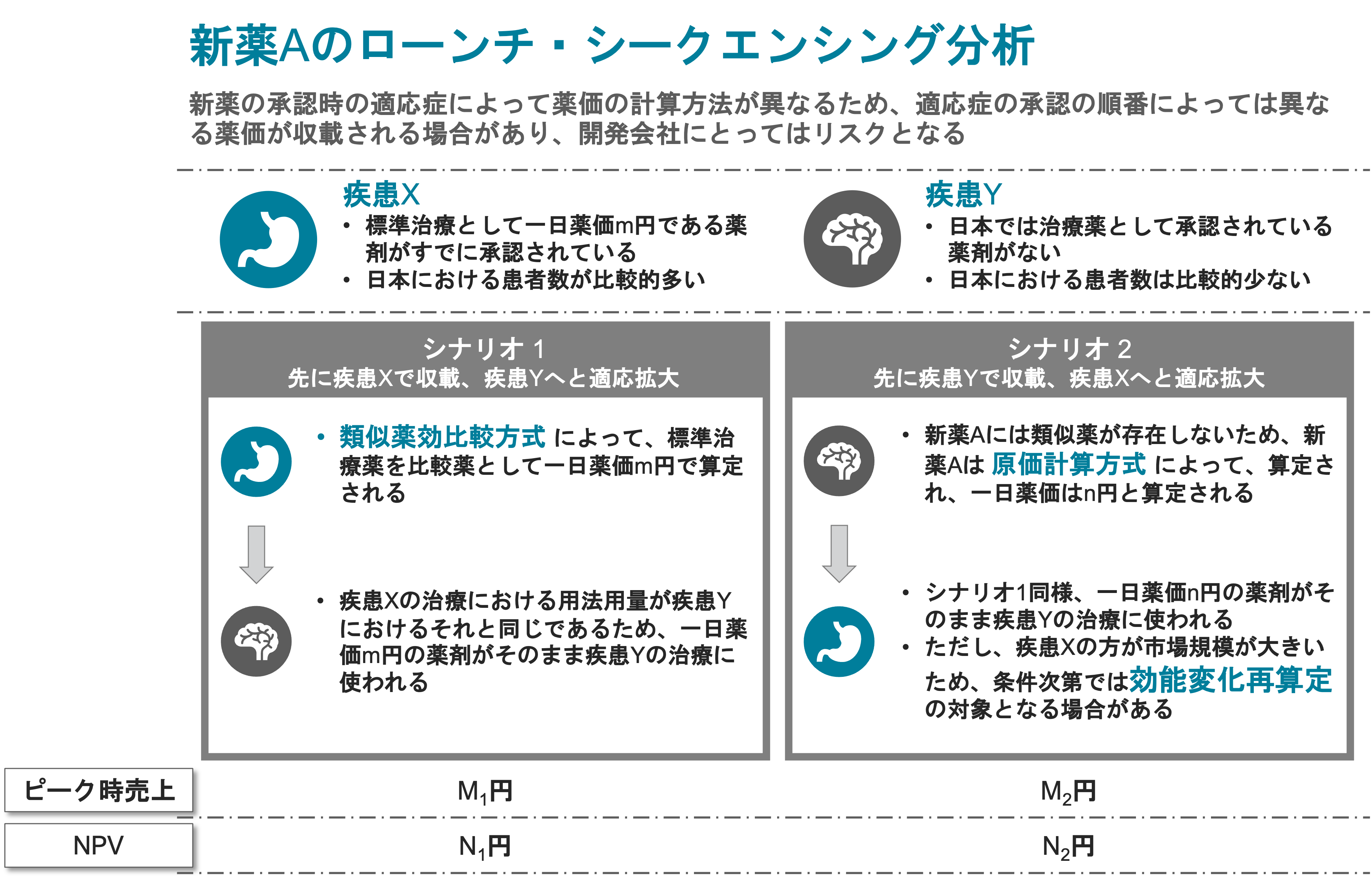
Understand Japanese market landscape for a severe, rare disease with only about 3,000 patients. Obtain feedback on product profile for multiple scenarios from KOLs in the pediatrician community in Japan.
Project Summary
- Analyzed market data and projected demand for drugs in the same category.
- Interviewed KOLs regarding opinions and preferences about product profiles. Identified product attribute with competitive advantage.
- Provided comprehensive estimation of NHI price for product developed in rare but debilitating disease space.
Suggested optimal development strategy for best price to maximize enterprise value of product. - Proposed local clinical development strategies aligned with global program and meeting local regulatory
requirements.
Client Feedback
Client was satisfied with our detailed evidence-based work. Our timelines met their need to quickly deliver valuation of their product to the investor community.
Timeline
12 weeks
Key Project Capabilities
- Demand Analysis
- Pricing and Market Access Consultation
- Market Research
- P&L Forecast/Valuation
- Clinical Development Consultation
Deliverables
- Excel-based forecasting model
Population-based
Multiple scenarios
Parametric
Reflects competitive landscape
Evidence/fact-based time series analysis using cutting edge algorithms - PowerPoint presentation
KOL comments, candid opinions about new product, minimal translation/process loss
Clear optimal development strategy and projection of product incremental value in Japan
We help non-Japanese pharma/biotech companies
understand and expand into the Japanese market.