Chuikyo approved listing of 14 Drugs on May 25
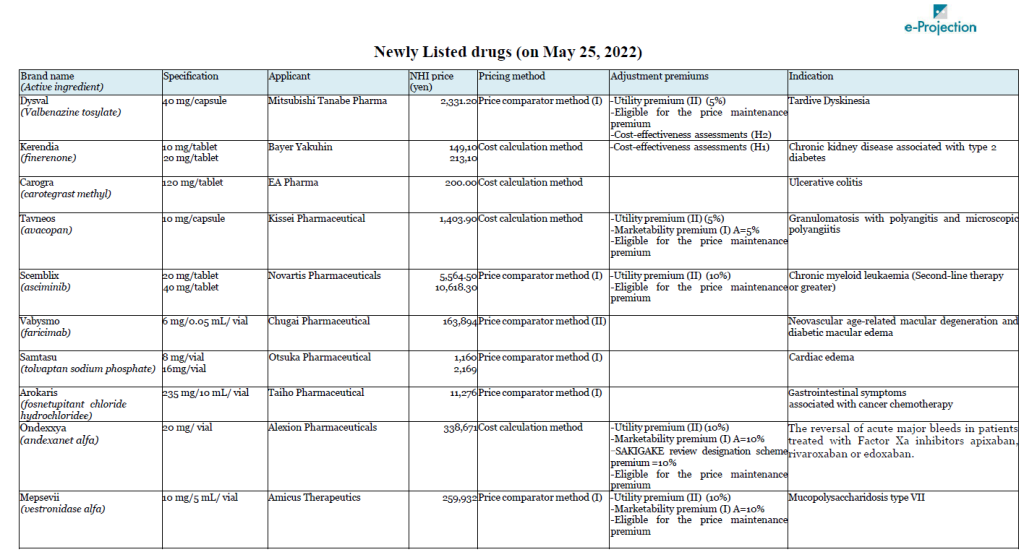
A total of 14 drugs will newly join Japan’s NHI price list on May 25. Chugai’s Vabysmo (faricimab), the first bispecific antibody in ophthalmology, for neovascular age-related macular degeneration and diabetic macular edema, is carrying the highest peak sales outlook among them, at 32 billion yen.
Bayer’s non-steroidal selective mineralocorticoid receptor antagonist, Kerendia (Finelenone) also has peak sales of over 10 billion yen. Bayer had submitted an objection to the initial proposal regarding the calculation method for the drug. As a result, the authorities gave an opinion that it is difficult to select an appropriate comparative drug, and the cost calculation method was applied.
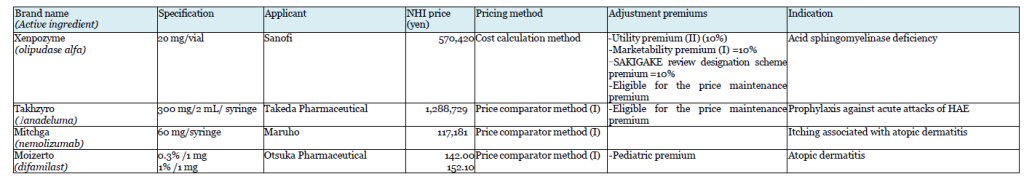
Takeda’s Takhzyro (lanadeluma) was also the subject of a negative opinion on the initial proposal. Takhzyro is indicated for “a prophylaxis against acute attacks of HAE,” and Orladeyo (berotralstat hydrochloride) was referred as the comparator drug in the calculation under the Price comparator method. Takeda appealed the applicability of Utility premium, citing the fact that the drug is the first-line drug in the guidelines in WAO/EAACI, U.S., and Canada, but Takhzyro was not awarded the premium.