New Drugs Added to NHI Price List on August 12
The Central Social Insurance Medical Council (Chuikyo), an advisory board for the reimbursement system relating to public healthcare insurance, endorsed the listing of 23 new drugs with 15 ingredients at its general meeting on August 4.
These new joined the NHI price list on August 12, including Chugai Pharmaceutical’s Evrysdi (risdiplam), which marks the first oral treatment for spinal muscular atrophy (SMA), as well as Gilead Sciences’ COVID-19 drug Veklury (remdesivir). Since Veklury earned special emergency approval in May last year, it has been purchased by the central government and its distribution has been under the control in order to ensure its fair allocation to patients in need. Veklury was priced by the cost-based method as it has no comparator medicine that has the same mechanism of action.
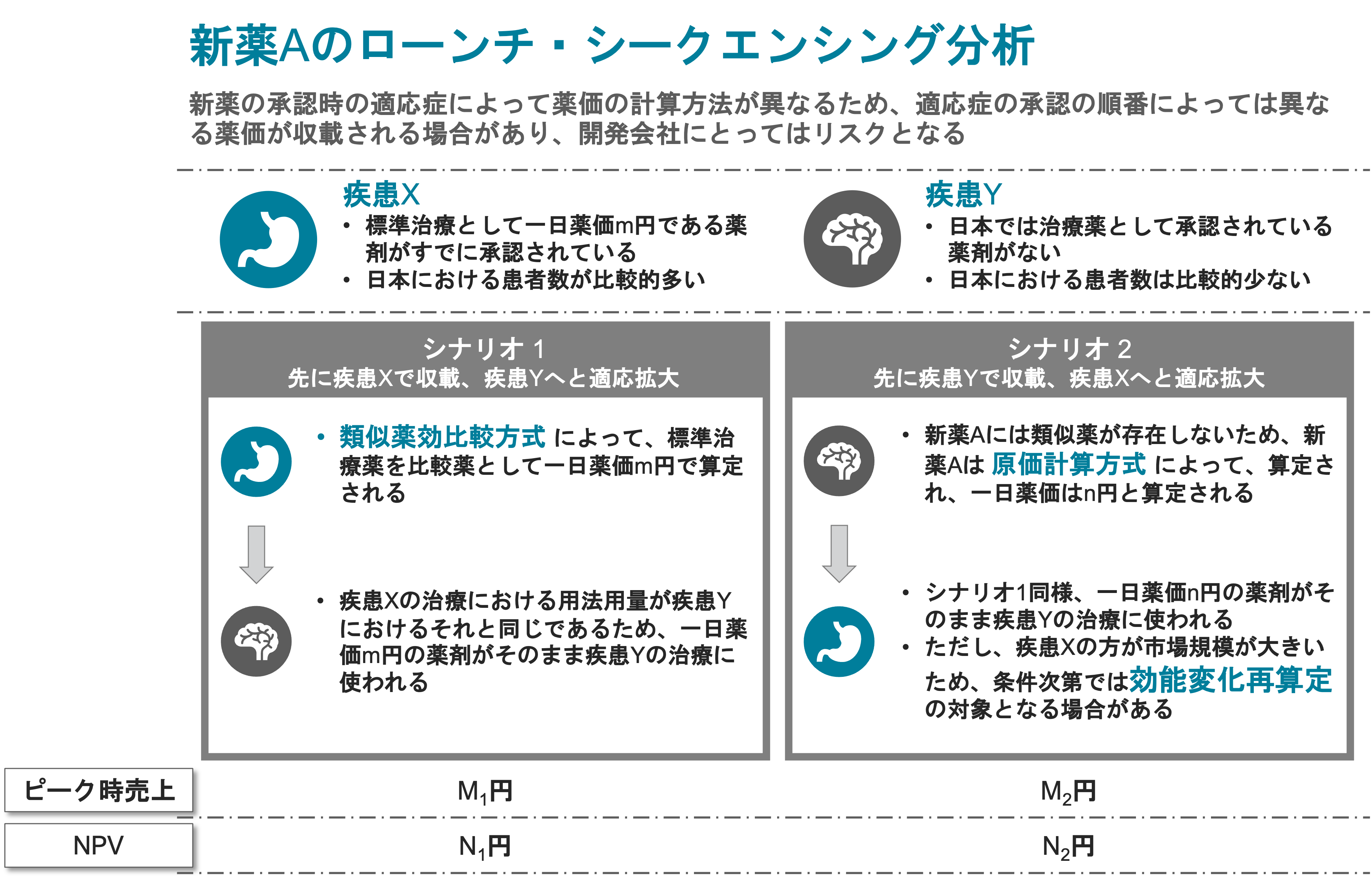
Newly Listed drugs (on August 12, 2021)
| No. | Brand name | Unit specification | Applicant | Active ingredient | Approval category | NHI price (yen) | Pricing method | Adjustment premiums etc. | Indication |
| 1 | EVRYSDI Dry Syrup | 60 mg/ bottle | Chugai Pharmaceutical Co., Ltd. | risdiplam | New active ingredient | 974,463.70 | Price comparator method (I) | Utility premium (II) A = 5 %
New drug development premium |
Spinal muscular atrophy |
| 2 | VERQUVO tablets | 2.5 mg/tablet 5 mg/tablet 10 mg/tablet |
Bayer Yakuhin, Ltd. | vericiguat | New active ingredient | 131.50 230.40 403.80 |
Price comparator method (I) | Cost-effectiveness assessments (H5) | Chronic cardiac insufficiency |
| 3 | TWYMEEG tablets | 500 mg/tablet | Sumitomo Dainippon Pharma Co., Ltd. | imeglimin hydrochloride | New active ingredient | 34.40 | Price comparator method (I) | – | Type 2 diabetes
|
| 4 | TAZVERIK tablets | 200 mg/tablet | Eisai Co., Ltd. | tazemetostat hydrobromide | New active ingredient | 3,004.60 | Price comparator method (I) | Utility premium (II) A=5%
New drug development premium |
Relapsed or refractory EZH2 gene mutation-positive follicular lymphoma (only when standard treatment is not applicable) |
| 5 | HIYASTA tablets | 10 mg/tablet | Huya Japan | tucidinostat | New active ingredient | 20,030.50 | Price comparator method (I) | New drug development premium | Relapsed or refractory adult T-cell leukemia-lymphoma (ATLL). |
| 6 | AJOVY Syringes for S.C. Injection 225mg | 225 mg/1.5 mL/syringe | Otsuka Pharmaceutical Co., Ltd. | fremanezumab (genetical recombination) |
New active ingredient | 41,356 | Price comparator method (I) | Cost-effectiveness assessments (H5) | Suppression of onset of migraine attacks |
| 7 | AIMOVIG Subcutaneous Injection Pens | 70 mg/1 mL/kit | Amgen Co., Ltd. | erenumab (genetical recombination) | New active ingredient | 41,356 | Price comparator method (I) | Cost-effectiveness assessments (H5) | Suppression of onset of migraine attacks |
| 8 | REVESTIVE 3.8mg for S.C.Injection | 3.8 mg/vial | Takeda Pharmaceutical Company Limited | teduglutide (genetical recombination) | New active ingredient | 79,302 | Cost calculation method | Utility premium (II) A=5%
Marketability premium (I) A=10% New drug development premium Cost-effectiveness assessments (H2) |
Short bowel syndrome |
| 9 | LYSAKARE Injection | 1 L/bag | Fujifilm Toyama Chemical Co., Ltd. | l-lysine hydrochloride and l-arginine hydrochloridee | New medical combination | 1,180 | Price comparator method (I) | – | Reduction of renal radiation exposure by lutetium (177Lu) oxodotreotide |
| 10 | GIVLAARI Subcutaneous Injection | 189 mg/1 mL/vial | Alnylam Japan Co., Ltd. | givosiran | New active ingredient | 5,006,201 | Cost calculation method | Utility premium (II) A=40 %
Marketability premium (I) A=10% New drug development premium |
Acute hepatic porphyria |
| 11 | UPASITA IV Injection Syringe for Dialysis | 25 μg/1 mL/syringe 50 μg/1 mL/syringe 100 μg/1 mL/syringe 150 μg/1 mL/syringe 200 μg/1 mL/syringe 250 μg/1 mL/syringe 300 μg/1 mL/syringe |
Sanwa Kagaku Kenkyusho Co., Ltd. | upacicalcet sodium hydrate | New active ingredient | 976 1,392 2,007 2,494 2,914 3,291 3,635 |
Price comparator method (I) | – | Secondary hyperparathyroidism in patients on hemodialysis |
| 12
|
LUTATHERA injection | 7.4 GBq/25 mL/vial | Fujifilm Toyama Chemical Co., Ltd. | lutetium (177Lu) oxodotreotide | New active ingredient | 2,648,153 | Cost calculation method | Utility premium (II)A=10%
New drug development premium |
Somatostatin receptor-positive neuroendocrine tumors |
| 13 | UNITUXIN I.V. injection 17.5mg/5mL | 17.5 mg/5 mL/vial | Ohara Pharmaceutical Co., Ltd. | dinutximab (genetical recombination) | New active ingredient | 1,365,888 | Cost calculation method | Utility premium (II)A=5%
Marketability premium (I)A=10% New drug development premium |
Neuroblastoma after high-dose chemotherapy |
| 14
|
RECARBRIO Combination for Intravenous Drip Infusion | 1.25 g/vial | MSD Co., Ltd. | relebactam hydrate
imipenem hydrate cilastatin sodium |
Drugs containing new active ingredients
New prescription combination drugs |
22,447 | Cost calculation method | Utility premium (II)A=5 %
Utility premium (II)A=10% New drug development premium |
Infections due to aerobic gram-negative organisms in adults with limited treatment options |
| 15 | VEKLURY for Intravenous Injection | 100 mg/vial | Gilead Sciences Inc | remdesivir | Specially Approved Pharmaceuticals.
(New active ingredient) |
63,342 | Cost calculation method | New drug development premium
Cost-effectiveness assessments (H1) |
SARS-CoV-2 infection |
Feel free to ask us any questions regarding NHI pricing method. Contact us: hikari.matsuda@e-projection.com